2. The Chemistry of Life
2.1
Elements of life
2.1.1
State that the three commonest elements of life are carbon, hydrogen and oxygen.
2.1.2 State that a variety of other elements are needed by living organisms including nitrogen, sulfur, phosphorus, iron and potassium.
2.1.3
State one role for each of the elements
mentioned in 2.1.2.
Refer to roles in plants and animals. An opportunity to emphasise Universality
2.1.4 Outline the difference between an atom and an ion.
Ions only in terms of being charged particles
ion - An atom or compound that has gained or lost one or more electrons, and hence has acquired an overall positive or negative charge.
2.1.5
Define organic.
2.1.6
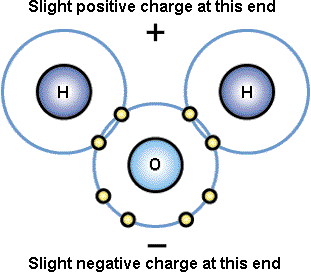
Outline the significance of water in biology including transparency, cohesion,
surface tension, solvent properties and thermal properties, referring to the
polarity of water molecules and hydrogen bonding where relevant.
Quantitative
details of bond angles, strengths, or electro-negativity, are not required.
Limited to the O 'side' of the (H2O) molecule being slightly negative
and the H 'side' being slightly positive. One example to illustrate the
importance of each property is sufficient. Thermal properties - refer to the
large amounts of energy required to heat up water and change its state (and the
reverse). Solvent properties - the fact that water is capable of dissolving many
organic and inorganic particles.

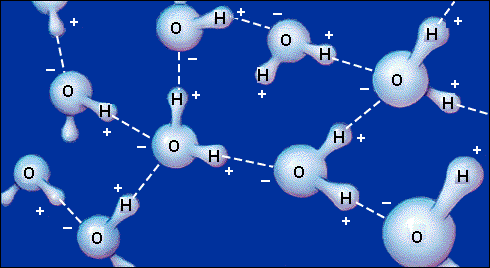
2.1.7 Discuss the significance of water to organisms as a coolant, transport medium and habitat, in terms of its properties.
Both
plants and animals should be mentioned. No physical, chemical or quantitative
details are required, only an outline.
2.2 Carbohydrates, lipids and
proteins
2.2.1
Draw the basic structure of a generalised amino acid.
Only
a 2-dimensional structural formula is required; no details of the R group.
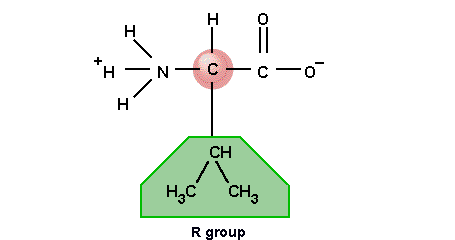
This is one example each amino acid differs only by the R group
2.2.2 Draw the ring structure of alpha-D-glucose.
2.2.3 Draw the basic structure of glycerol and a generalised fatty acid.
2.2.4
Outline the role of condensation and hydrolysis in the relationships
between monosaccharides and disaccharides; fatty acids, a glycerol and
triglycerides; amino acids, dipeptides and polypeptides.
Limited
to the breaking of covalent bonds by H+ / OH- and the
making of covalent bonds, with the resulting formation of H2O
molecules.
2.2.5
Draw the structure of a generalised dipeptide, showing the peptide
linkage.
Neither
the fact the linkage is planar nor that it permits rotation about the C-N bond
is required.
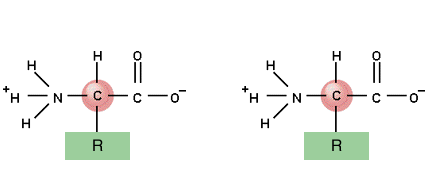
Two amino acids

The bond occurs between the Carboxyl group and the amino group
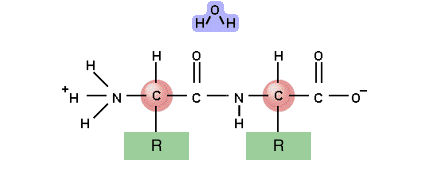
Water is produced from an O from the carboxyl group and 2 H's from the amino group - CONDENSATION

The bond forms between the C of the carboxyl group and the N of the amino group
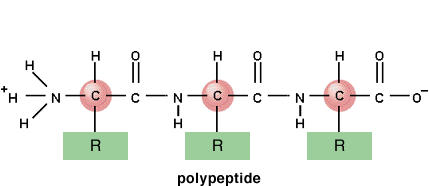
and so the chain continues - lots of peptide bonds between lots of amino acids gives lots of water and a polypeptide
2.2.6
Explain the relative solubility of carbohydrates, lipids and protein in
water.
Limited
to the general insolubility of most lipids due to the long hydrocarbon chain
(hydrophobic). The general solubility of carbohydrates being due to the presence
of OH groups (hydrogen bonding again) and proteins being due to the presence of
these groups, other groups and charges. Mention that solubility tends to
decrease with increasing size.
2.2.7
Compare the energy content of carbohydrates, lipids and proteins.
Qualitative
comparisons only, lipids having about mice the energy content of both proteins
and carbohydrates (by mass).
2.2.8
List two examples each of
monosaccharides, disaccharides and
polysaccharides.
Only
the names and the names of the monomer units are required, not structural
formulae.
2.2.9
State one function for a monosaccharide and one for a polysaccharide.
2.2.10
State three functions of lipids.
2.3 Enzymes
2.3.1
Define enzyme.
23.2
Define active
site.
2.3.3
Describe the "lock-and-key" model.
2.3.4
List three factors that affect enzyme activity.
Any
three factors are permissible, but should probably include temperature and
substrate concentration, since they are specifically mentioned in 2.3.5.
2.3.5
Outline the effects of temperature and substrate concentration on enzyme
activity.
2.3.6
Define
denaturation.
2.3.7
Explain two applications of enzymes in biotechnology.
Applications
could include oil digesting bacteria, bacterial extraction of metals from ores,
yoghurt, cheese, biological washing powder and tenderizing meat. Many more
examples will develop over the life of the programme. Detailed chemistry is
not expected, but reasons for the use of biotechnology as well as the
advantages conferred by it is required.
2.4 DNA structure
2.4
.1 Outline DNA nucleotide structure in terms of sugar (deoxyribose), base
and phosphate.
Chemical
formulae
2.4.2
State the names of the four bases in DNA.
2.4.3
Outline how the DNA nucleotides are linked together by covalent bonds
into a single strand.
2.4.4
Explain how a DNA double helix is formed using complementary base
pairing, and hydrogen bonds.
2.4.5
Draw a simple diagram of the
molecular structure of DNA.
An extension of the diagram above is sufficient. A 'twisted ladder'
arrangement to show, the
2.5 DNA replication
2.5.1
State that DNA replication is semi-conservative.
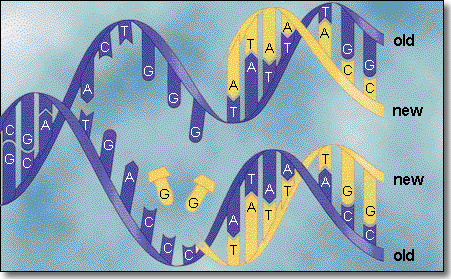
2.5.2
Outline DNA replication in terms of unwinding the double helix and
separation of the strands by helicase followed by formation of the new
complementary strands by DNA polymerase.
It
is not necessary to mention the fact that there is more than one helicase and
polymerase or that more enzymes are involved.
|
If you want to see a little more detail than stated here - Go to the Nucleic Acids and Proteins AHL page |
2.5.3
Explain the significance of complementary base pairing in the
conservation of the base sequence of DNA.
2.6 Transcription and translation
2.6.1
Compare the structure of RNA and DNA.
Limit
it to names of sugars, bases and number of strands.
2.6.2
State one function of messenger RNA and one function of transfer RNA.
2.6.3
Outline DNA transcription in terms of the formation of a RNA strand
complementary to the DNA strands by RNA polymerase.
2.6.4
Describe the genetic code in terms of codons composed of triplets of
bases.
2.6.5
Describe translation including the roles of mRNA codons, tRNA anticodons
and ribosomes leading to peptide linkage formation.
Mention of the location, other enzymes, energy source or rate is not
required.
2.6.6
Define the terms degeneracy and
universal as they relate to the genetic code.
2.6.7
Explain the relationship
between one gene and one polypeptide and its significance.
2.7 Genetic engineering, DNA fingerprinting, gene therapy
2.7.1
State that genetic material can be transferred between species because
the genetic code is universal (cross reference 2.6.6).
2.7.2 Outline a basic technique used for gene transfer involving plasmids, a host cell (bacterium, yeast or other cell), restriction enzymes (endonuclease) and DNA ligase.
The
use of E. coli in gene technology is
well documented. Most of its DNA is in one circular chromosome but it also has
plasmids (smaller circles of DNA helix). These plasmids can be removed and
cleaved by restriction enzymes at target sequences. DNA fragments from another
organism can also be cleaved by the same restriction enzyme and these pieces can
be added to the open plasmid and spliced together by ligase. The recombinant
plasmids formed can be inserted into new host cells and cloned.
2.7.3
State two examples of the current uses of genetic engineering in
agriculture and/or pharmacy.
Improved
crops and animal breeds by increased disease resistance, heavy metal tolerance
and better yield can be mentioned. Bacteria can be made to manufacture useful
compounds for various purposes, for example insulin.
It
is an attractive teaching point to emphasise the concentrated time scale in the
development of genetic manipulation techniques.
1953
Watson and Crick- DNA model
1970
first restriction enzymes
1973
plasmid splicing
1977
first engineered bacteria
1985
genetic fingerprinting
1989
cystic fibrosis gene cloned and sequenced
1994
generically manipulated organisms for food
2.7.4
Explain one potential harmful result of genetic engineering.
Some controversial gene transfers are regarded as harmful, by some people (e.g. bovine sornatotropin). A possible problem exists with the release of genetically engineered organisms in the environment. They could spread and compete with the naturally occurring varieties. Some of the engineered genes could also cross species barriers.
2.7.5
State that PCR (polymerase chain reaction) copies and amplifies minute
quantities of nucleic acid.
Details
of Methods are not required.
Check out this excellent PCR website
2.7.6
State that gel electrophoresis involves the separation of fragmented
pieces of DNA according to their charge and size.
2.7.7
State that gel electrophoresis of DNA is used in DNA profiling.
2.7.8
Describe two applications of DNA profiling.
Applications
could include some criminal investigation such as murder or rape, or paternity
suits. The identification of people that died a long time ago, or information
about them is possible (e.g., the dead Tsars of Russia and some Egyptian
mummies). Draw attention to the problems of contamination of samples.
2.7.9
Outline the process of gene therapy using, a named example.
This
involves replacement of defective genes. White blood cells or bone marrow cells
are removed and, by means of a vector, the normal gene is introduced and inserted into the chromosome. The cells are
replaced in the patient so that the normal gene can be expressed. Examples are
the use in cystic fibrosis and SCID (a condition of immune deficiency, where the
replaced gene allows for the production of the enzyme ADA - adenosine
deaminase). A cure for thalassaemia is also possible